New Synthetic Adhesives Derived from… Oysters?
I bring this up because tasting good (to other people, at least) is a positive characteristic of oysters. Another positive trait is their uncanny ability to filter dirty nastiness out of rivers and bays, clarifying the water in which they dwell alongside myriad other creatures. But from an architect’s standpoint, the most superlative characteristic of an oyster has to be its ability to stick itself to fellow oysters and riverbeds, enabling the formation of complex, long-lasting reefs. These large oyster accumulations provide habitat for other species and protect the coastline from the ravages of storms. And until recently, human beings had no clear idea exactly how oysters accrete to one another.
Image courtesy www.seattlemet.com
Image courtesy www.climateshift.org
At Purdue University, a research team has conducted experiments to discover the unique properties and composition of oyster cement, a “biomineralized adhesive material for aggregating into large communities. This cement is an organic-inorganc hybrid and differs from the surrounding shells by displaying an alternate CaCO3 crystal form, a cross-linked organic matrix, and an elevated protein content … the high inorganic content is exclusive to oysters” (Burkett et al). If your eyes began to cross while reading the previous quote, have someone slap you on the back, have a shot of espresso, and stay with me.
Image copyright Burkett, et al. Department of Chemistry and School of Materials Engineering, Purdue University
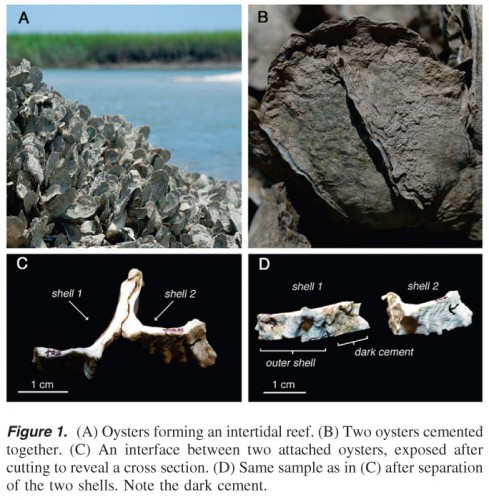
The researchers cut small sections through oyster shells that had attached to each other via a small band of gray material that was still visible between the shells. They pulverized samples of the outer shell, the gray material (oyster cement) and the inner shell of the oysters, and subjected each type of powder to various tests. They dehydrated it, they treated it with acid, and they used Infrared (IR) spectroscopy among other means to determine that, in fact, the inner shell and outer shell of the oysters were compositionally distinct from the oyster cement. Further, “the researchers were able to determine that the adhesive contained almost five times the amount of protein than what is found in the shell, as well as both iron and highly oxidized, cross-linked proteins” (Materials Technology). Aaaand boom goes the dynamite!
What this means is that the oyster adhesive is more inorganic than your typical hydrated, organic glue-like material produced by mussels and barnacles (which are, admittedly, quite strong). Those adhesives are composed mainly of proteins whereas oyster adhesive consists of about 90 percent calcium carbonate (chalk). When I looked into calcium carbonate on the interwebs, I found out that the substance is crazy commonplace all over the globe – it’s found in all kinds of shells, most rocks, and even snails.
It’s thought by the Purdue research team that what makes the oyster cement so effective is the interaction between the calcium carbonate and the small amount of protein that binds everything together. They’re planning to “investigate the interaction of the different components within oyster cement and use this information for developing new synthetic materials” (Materials Technology). If they can unravel the inner workings of oyster cement they’ll be able to “provide blueprints for the design of biomimetic materials, aid development of adhesion-inhibiting antifouling surfaces, and illustrate the workings of healthy ecosystems” (Burkett et al). It’s exciting to think that we could be on our way to developing adhesives for use in medicine and construction that set and hold in wet environments.
*Do oysters even have eyes?? Gross.
Image courtesy www.content.cdlib.org
I am filing oyster cement under water (heh – get it!) and also in the earth category because you can basically make land if you have enough oysters. The Purdue paper mentioned that some reef structures are “tens of meters deep and several square kilometers in area” (Burkett et al).
Cited:
Burkett, Jeremy R., Lauren M. Hight, Paul Kenny, and Jonathan J. Wilker. “Oysters Produce and Organic-Inorganic Adhesive for Intertidal Reef Construction.” Journal of the American Chemical Society, 2010. Volume 132, pages 12531-12533.
“Oysters Offer Clues to New Adhesive Materials” Materials Technology @ TMS. 09/23/10. Accessed 10/05/10. URL.

















I know this is slightly off-topic, but I doubt anyone likes the taste of oysters. They like the experience of oysters which is, like downing a shot of vodka with friends, both disgusting and strangely life-affirming. Or not.
[…] year I wrote about the adhesive they use to adhere themselves to this and that underwater object (read the post here) and I will admit that since the time of that writing I ate one. That’s right people – I […]
Leave a Wordpress Comment:
Ads
Watch ARCHITERIALS Videos on vimeo
Like on Facebook
Twitter
Flickr
Hit Counter
Ads
Blogs
Green
Journals/Publications
Materials
Network/News
Offices/People
Resources
Science
Pages
Archive
RSS and Email Subscriptions
Tag Cloud
3D 3D printer AB FAB academic acid acrylic actuated matter adaptive adhesive adsorption aerogel air air conditioning alloy aluminum amnh antibacterial antifungal ants april fool's architecture architecture robot artificial skin autonomous aviation awesome bacteria bamboo bananas beer bench bend bending biennale biocomputing biodegradable biodegradeable biomaterials biomimetics biomimicry biominerals biopolymer birds blast blast-resistant block blocks blogs Bloom Box brazil brick bubbles bucky bulk metallic glass butterfly calera canvas carbon carbon fiber carbon nanotubes carpet cars ceiling cellulose cement ceramic chain link chair charcoal charlie sheen chemicals chiller clay cloth cloud cmu coils color color-changing communication compound computer concrete condensation conducting conductive context cool coral cracks crystal cyborg demakersvan design digifab dirt disaster dna dror drywall dutch dynamic EAP earth ecocradle ecolect e coli ecology ecoresin ecovative elastic electric electricity electrochromic electroluminescent electronic energy energy recovery environment evaporative cooling experiment fabric fabrication facade fiber fiberglass fiber optic fiber optics fibers film FIRE flexible flickr fly ash foam fungus furniture garbage gel geodesic dome geometry gfrp gilgamesh glass glass fiber glow glue gold graphene green greensulate gsapp gypsum hard heat heavy heidi klum helix hemp hexagon hidden high performance hive honeybee humidity ice India ink insulation interference Internet inventables invisible invisible ink jello jellyfish just add water kevlar kinetic korea lace lamboo laser lattice leaves LED leed LEGO light light emitting light transmitting liquid lo mein london Loop.pH machines magic magnetic marine material materials meatball melting memory metabolic engineering METAL metal panel metamaterial micro microsensor microtools military milk MIT moisture multi-layer mushrooms mycelium nano nanogel nanotech nanotubes NASA new noise non-metallic oil OLED OMA ostrich oysters packaging paint panel panels paper paperfoam paraffin wax particles particulates petroleum phase change phosphorescent pink PLA plastic platinum pm-10 poetry pollution polymer polymers porcelain power precast printed printing protein public quadror radiant rain rammed earth reclaimed recycled reflective refracting Rem Koolhaas resin robot robotics roof rubber rugged sand sealant sealer search segmented self-healing sensor shape memory silica silicon silk skin skittles slats smog soft solar solar cell solar cells solar paint solid solid state lighting sound spider spider glue spray stabilized sand stone stretch stretchable strong structural structure studio dror sun sunglasses super supercritical sustainable switzerland tags tape technology TED tensile TEXAS textile textiles texture thermal thermochromatic thin thin film thread tiger stone tile tiles timber tio2 tires toaster tokujin yoshioka touch touch-sensitive toxic transparent t shirts tulip ultra-thin university of akron university of connecticut upcycling virus voc wall wallpaper WATER web wet whale wires WOOD woodwool wool workshop
WP Cumulus Flash tag cloud by Roy Tanck and Luke Morton requires Flash Player 9 or better.
Recent Comments
Ads